How to Read Electron Configuration of Molecular Orbital
Learning Objectives
- Proceeds an understanding of molecular orbital theory.
- Learn to summate bond orders.
- Learn to draw molecular orbital electron configuration free energy diagrams.
Valence bail theory is able to explain many aspects of bonding, but not all. To complement this theory, we utilise another called the molecular orbital (MO) theory. Molecular orbital theory is a more than sophisticated model for understanding the nature of chemical bonding.
MO theory takes the idea of atomic orbitals overlapping to a new level, where new molecular orbitals are generated using a mathematical process chosen linear combination of atomic orbitals (LCAO).
Molecular orbitals share many similarities with atomic orbitals:
– They are filled from lowest free energy to highest energy (Aufbau principle).
– They tin hold a maximum of two electrons of reverse spin per orbital (Pauli exclusion principle).
The major difference between diminutive and molecular orbitals is that atomic orbitals stand for electron density in infinite associated with a particular cantlet. Molecular orbitals are associated with the entire molecule, meaning the electron density is delocalized (spread out) over more than one cantlet.
The Molecular Orbitals of the Hydrogen Molecule
Combining the 1s orbitals of each hydrogen atom using LCAO, two molecular orbitals are generated σ1south (pronounced sigma one southward) and σ*onesouth (pronounced sigma star one s).
The σis orbital is generated by a effective combination (or interference), where the two atomic orbitals wave functions reinforce (add to) each other. This is the lower energy of the two molecular orbitals and is known every bit the bonding molecular orbital. Detect in Figure ix.nineteen "Hydrogen molecular orbital combination diagram"that the electron density of this orbital is concentrated between the two nuclei. These electrons are stabilized past attractions to both nuclei, and they concur the atoms together with a covalent bond.
The σ*1s orbital is generated by a destructive combination (or interference), where the moving ridge functions of the two atomic orbitals abolish each other. This type of combination results in an surface area of zero electron density betwixt the two nuclei, known as a nodal plane (or node). This node of cypher electron density is destabilizing toward the bail, making information technology higher free energy, and subsequently this type of orbital is known as an antibonding molecular orbital (denoted by the asterisk in the orbital name).

Figure ix.19. Hydrogen molecular orbital combination diagram.
Similar to atomic orbitals, we tin can write electron configuration energy diagrams for molecular orbitals (Figure ix.20 "Hydrogen molecular orbital electron configuration energy diagram"). Notice that the atomic orbitals of each atom are written on either side, and the newly formed molecular orbitals are written in the centre of the diagram. The bonding molecular orbital is filled and is relatively lower in energy than the contributing diminutive orbitals, supporting the fact that hydrogen molecules (H2) are more than stable than lone hydrogen atoms.

Figure 9.20. Hydrogen molecular orbital electron configuration energy diagram.
Bond Order
Nosotros have just seen that the bonding molecular orbital is lower energy and promotes the formation of a covalent bond, while the antibonding molecular orbital is college free energy with a node of zero electron density between the atoms that destabilizes the formation of a covalent bond. We tin evaluate the strength of a covalent bail by determining its bond order.
Bail order = 1/2 (# of electrons in bonding MOs – # of electrons in antibonding MOs)
Bail-guild values can be whole numbers, fractions, or zero. These values correspond to the valence bond model, and then a bail order of 1 is equal to a single bond, and ii is equal to a double bail. A value of naught ways that at that place is no bail present, and the atoms be separately.
Example 11
Decide the bond club of the hydrogen molecule.
Solution
Bond social club = 1/2 (# of electrons in bonding MOs – # of electrons in antibonding MOs)
Bail social club = ane/2 (two – 0) = one
Therefore there is a single bond in the hydrogen molecule.
Molecular Orbitals of Li2
Generating molecular orbitals of molecules more than complex than hydrogen using the LCAO method requires following a few additional guidelines:
– The number of MOs generated is equal to the number of atomic orbitals combined.
– Combined atomic orbitals should exist of similar energy levels.
– The effectiveness of atomic orbital combination depends on the amount of orbital overlap. Increased overlap lowers the energy of the bonding molecular orbital further, and raises the energy of the antibonding molecular orbital.
Let'south follow these guidelines and generate a molecular orbital electron configuration diagram for Liii(Figure 9.21 "Molecular orbital electron configuration energy diagram for dilithium"):

Figure nine.21. Molecular orbital electron configuration free energy diagram for dilithium.
Find that nosotros have combined the isouth atomic orbitals, as before in the H2 example, to generate bonding and antibonding molecular orbitals that are completely filled past both atoms' 1s electrons. Similarly 2due south atomic orbitals combine, giving a bonding orbital and an antibonding orbital, which are filled with the remaining valence electrons starting from the bottom up. The atomic orbitals that combine are of similar energy levels; a 1s orbital does not combine with one of the 2south orbitals.
The bond society can be determined for this molecule to be:
Bail society = 1/two (4 – two) = 1
Therefore Liii would have a single bond.
Molecular Orbitals from p Diminutive Orbitals
To determine the molecular orbitals of many other molecules, we demand to examine how p orbitals combine to give molecular orbitals. Thep orbitals can overlap in 2 ways: caput-to-caput or sideways. Head-to-caput overlap of p atomic orbitals results in a bonding and antibonding molecular orbital, where the electron density is centred along the internuclear axis, making them σ orbitals (Figure 9.22 "Caput-to-head overlap ofp orbitals").

Effigy nine.22. Head-to-caput overlap of p orbitals.
Sideways overlap of the remaining 4 p atomic orbitals can occur along the two other axes, generating four π molecular orbitals having electron density on reverse sides of the internuclear axis (Effigy 9.23 "Sideways overlap ofp orbitals").

Figure nine.23. Sideways overlap of p orbitals.
The head-to-caput overlap giving σ molecular orbitals results in greater overlap, making its bonding molecular orbital the most stable and lowest free energy, while the σ* antibonding is least stable and has the highest energy (Figure 9.24 "Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of diminutive number 8-10″). Sideways overlap gives 4 π molecular orbitals, two lower-energy degenerate-bonding molecular orbitals, and two higher-free energy antibonding orbitals.

Effigy 9.24. Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-x.
The energy diagram we have just generated fits experimentally with O2, F2, and Netwo, merely does not fit for Btwo, C2, and Nii. In the latter, homonuclear diatomic molecules (B2, Cii, and Ntwo), interactions take identify between the 2due south and 2p atomic orbitals that are strong plenty to swap the ordering of the σtwop and π2p molecular orbitals (Figure 9.25).
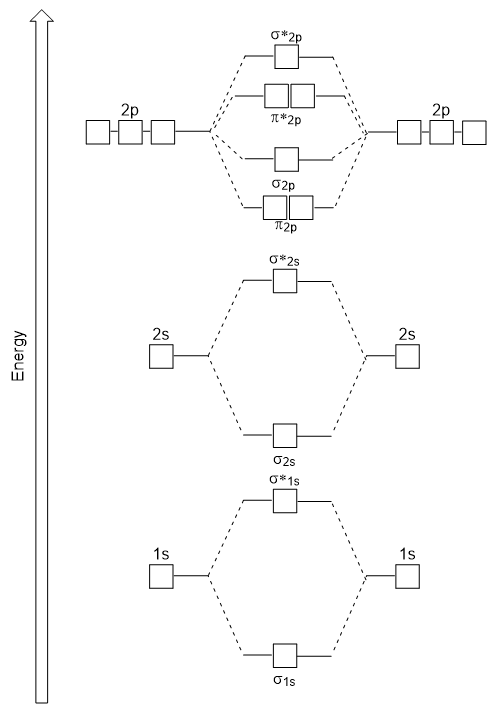
Figure 9.25. Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 5-vii.
Heteronuclear Diatomic Molecules
In heteronuclear diatomic molecules, where two dissimilar molecules are bonded, the energy levels of the individual atoms' diminutive orbitals may differ. However, the molecular orbital diagram nosotros see in Figure nine.25 ("Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of diminutive number 5-7″) can be used to estimate the electron configuration and bail order.
Frontier Molecular Orbitals
We tin can focus further on ii very important types of molecular orbitals: the highest occupied molecular orbital (Homo) and the lowest unoccupied molecular orbital (LUMO), as well referred to collectively as the frontier molecular orbitals (Effigy nine.26 "Frontier molecular orbitals Human being and LUMO"). Equally their names imply, the Homo is the molecular orbital that has the highest energy and contains electrons, while the LUMO is the everyman energy molecular orbital that does not contain electrons.

Effigy nine.26. Frontier molecular orbitals Human being and LUMO.
When molecules blot energy, information technology is typical for a HOMO electron to employ this energy to transition from the ground Human orbital to the LUMO excited-state orbital. This type of transition can exist observed in ultraviolet-visible (UV-Vis) radiations spectroscopy experiments. Every bit well, in many chemical reactions, one reactant molecule may donate HOMO electrons to the LUMO of another reactant (Figure nine.27 "Formation of a new bonding molecular orbital by combining reactant HOMO and LUMO"). Therefore, understanding frontier molecular orbital free energy levels can provide chemists with a swell deal of insight in the areas of molecular spectroscopy and reactivity.

Figure nine.27. Formation of a new bonding molecular orbital by combining reactant HOMO and LUMO.
Key Takeaways
- Diminutive orbitals can combine to make bonding and antibonding molecular orbitals.
- Bonding orbitals are lower in free energy than antibonding orbitals.
- Molecular orbitals are filled using like principles to atomic orbitals.
- Bond social club can be used to evaluate bond forcefulness.
- Frontier molecular orbitals are of particular importance in molecular spectroscopy and reactivity.
Source: https://courses.lumenlearning.com/suny-introductory-chemistry/chapter/molecular-orbitals/
Post a Comment for "How to Read Electron Configuration of Molecular Orbital"